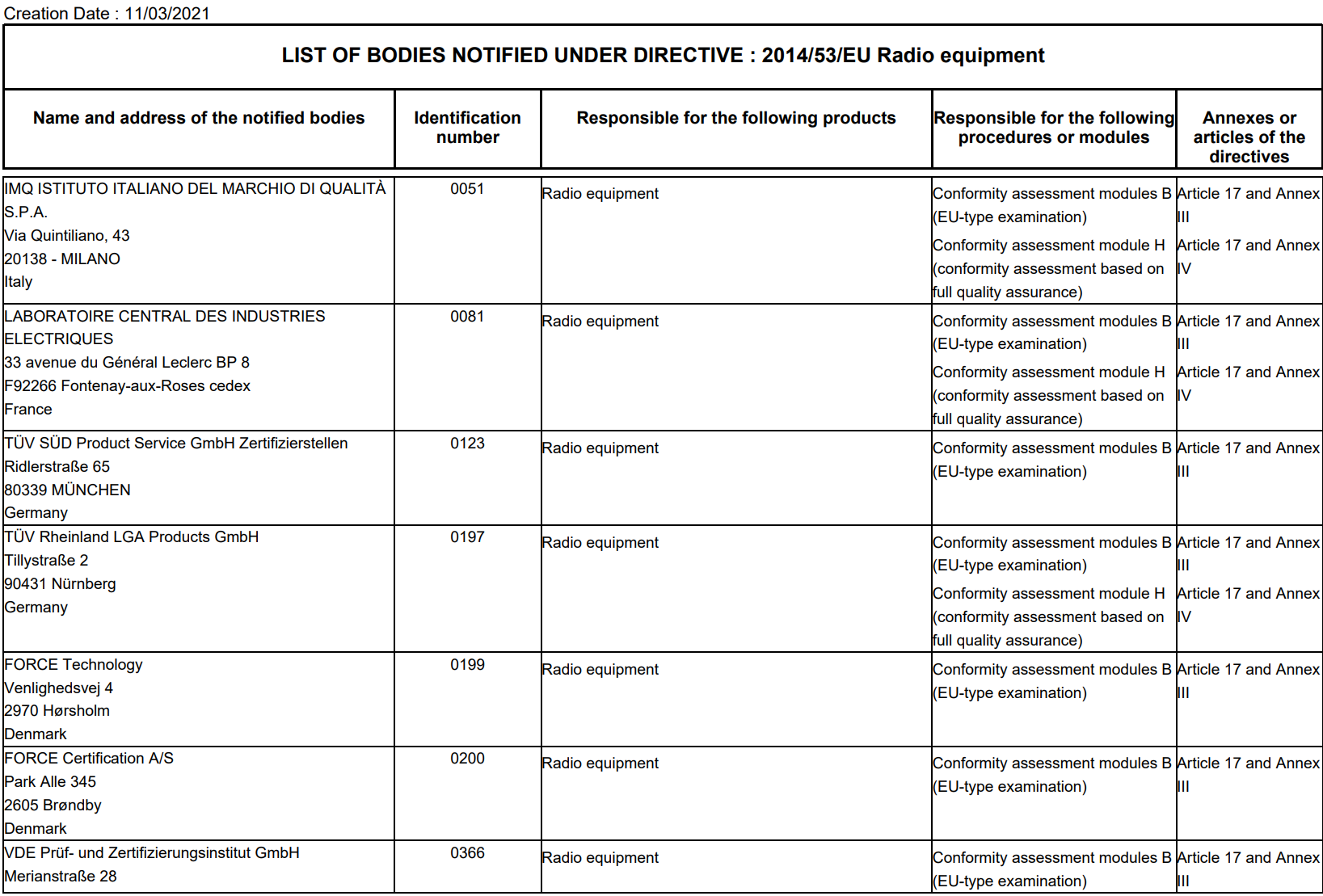
MDR: 26 Notified Bodies on NANDO & Swiss economic operator's requirements updated! · MDlaw – Information platform on European medical device regulations

Designation process of MDR/IVDR Notified Bodies - update · MDlaw – Information platform on European medical device regulations